Synthesis, Photophysical and Electronic Properties of a D-π-A Julolidine-Like Pyrenyl-o-Carborane
- Johannes Krebs, Lisa Brändler, Ivo Krummenacher, Alexandra Friedrich, Holger Braunschweig, Maik Finze, Basile F. E. Curchod and Todd B. Marder
- Publication
- May 17, 2024
Abstract:
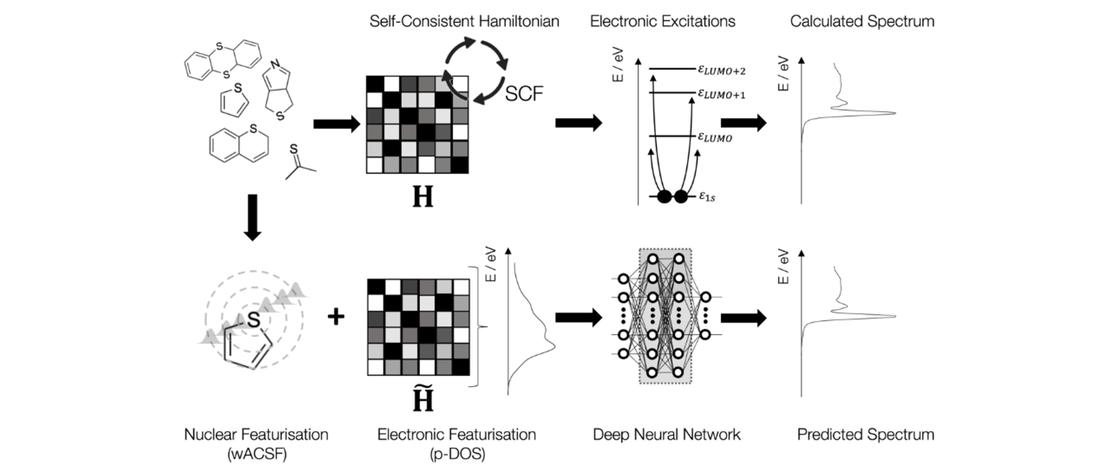
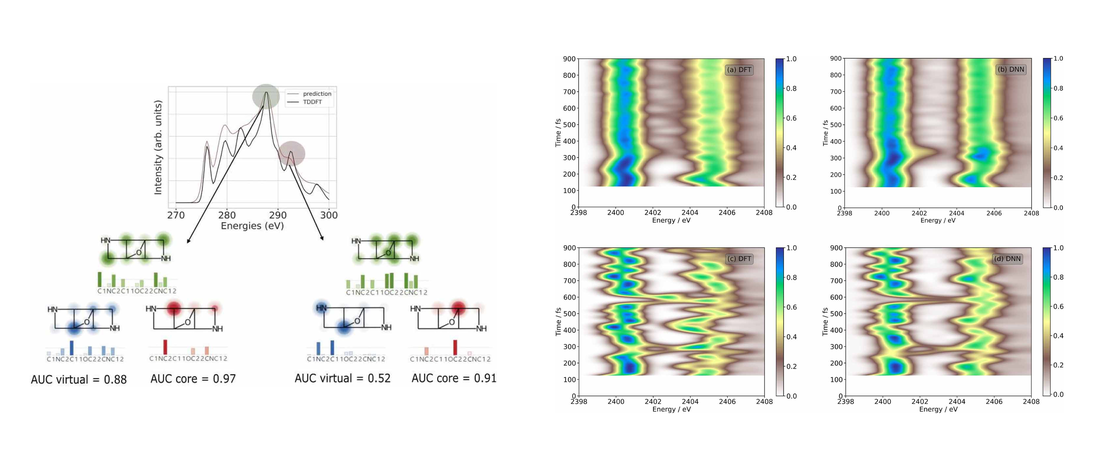
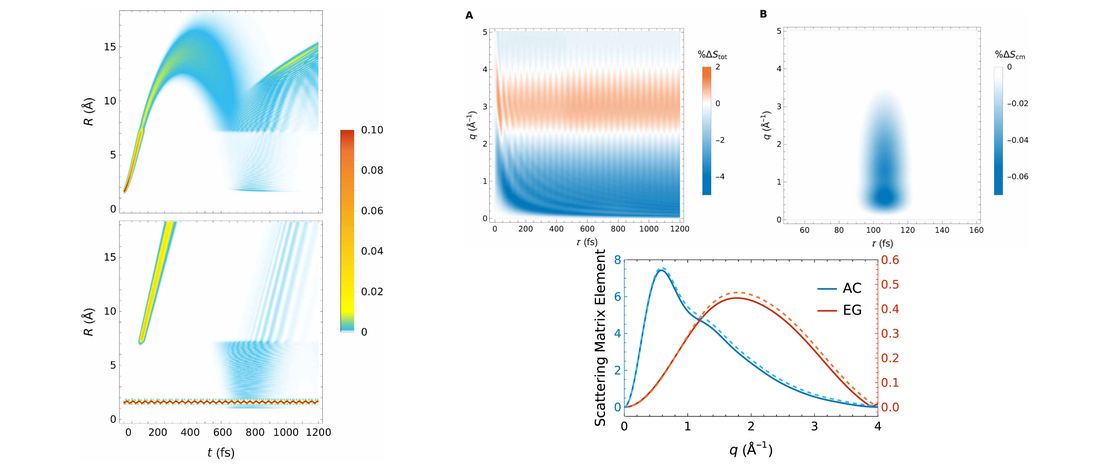
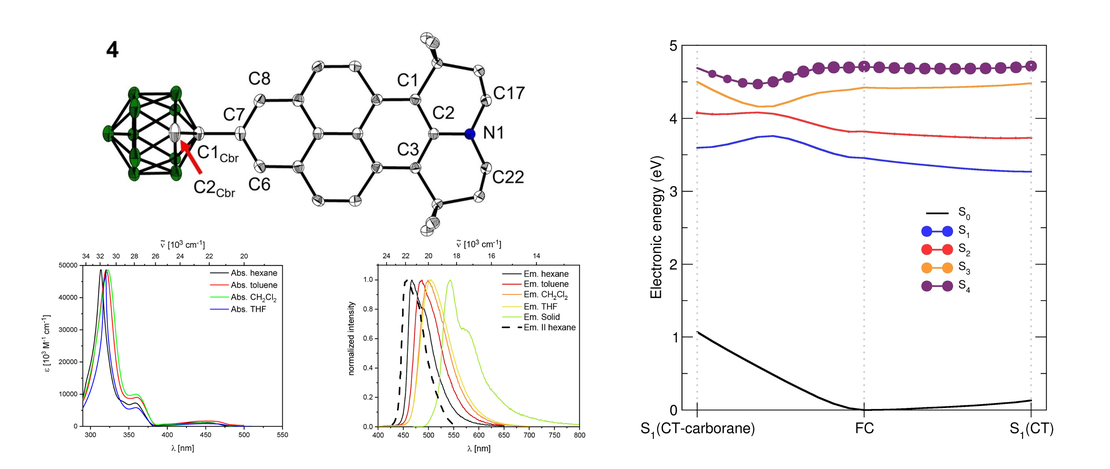
We synthesized 2-(1-1,2-dicarbadodecaboranyl(12))-6,6,12,12-tetramethyl-7,8,11,12-tetrahydro-6H,10H-phenaleno[1,9-fg]pyrido[3,2,1-ij]quinoline (4), a julolidine-like pyrenyl-o-carborane, with pyrene substituted at the 2,7-positions on the HOMO/LUMO nodal plane. Using solid state molecular structures, photophysical data, cyclic voltammetry, DFT and LR-TDDFT calculations, we compare o-carborane and B(Mes)2 (Mes=2,4,6-Me3C6H2) as acceptor groups. Whereas the π-acceptor strength of B(Mes)2 is sufficient to drop the pyrene LUMO+1 below the LUMO, the carborane does not do this. We confirm the π-donor strength of the julolidine-like moiety, however, which raises the pyrene HOMO-1 above the HOMO. In contrast to the analogous pyrene-2-yl-o-carborane, 2-(1-1,2-dicarbadodecaboranyl(12))-pyrene VI, which exhibits dual fluorescence, because the rate of internal conversion between locally-excited (LE) and charge transfer (CT) (from the pyrene to the carborane) states is faster than the radiative decay rate, leading to a thermodynamic equilibrium between the 2 states, 4 shows only single fluorescence, as the CT state involving the carborane as the acceptor moiety in not kinetically accessible, so a more localized CT emission involving the julolidine-like pyrene moiety is observed.
Additional Resources
DOI: 10.1002/chem.202401704
Bibtex:
@article{https://doi.org/10.1002/chem.202401704,
author = {Krebs, Johannes and Brändler, Lisa and Krummenacher, Ivo and Friedrich, Alexandra and Braunschweig, Holger and Finze, Maik and Curchod, Basile F. E. and Marder, Todd B.},
title = {Synthesis, Photophysical and Electronic Properties of a D-π-A Julolidine-Like Pyrenyl-o-Carborane},
journal = {Chemistry – A European Journal},
volume = {30},
number = {41},
pages = {e202401704},
keywords = {Carborane, Pyrene, Fluorescence, Charge transfer, DFT and LR-TDDFT calculation},
doi = {https://doi.org/10.1002/chem.202401704},
url = {https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.202401704},
eprint = {https://chemistry-europe.onlinelibrary.wiley.com/doi/pdf/10.1002/chem.202401704},
year = {2024}
}