XFEL SASE pulses can enhance time-dependent observables
- Eirik M Liane, Mats Simmermacher, Peter M Weber and Adam Kirrander
- Publication
- November 8, 2024
Abstract:
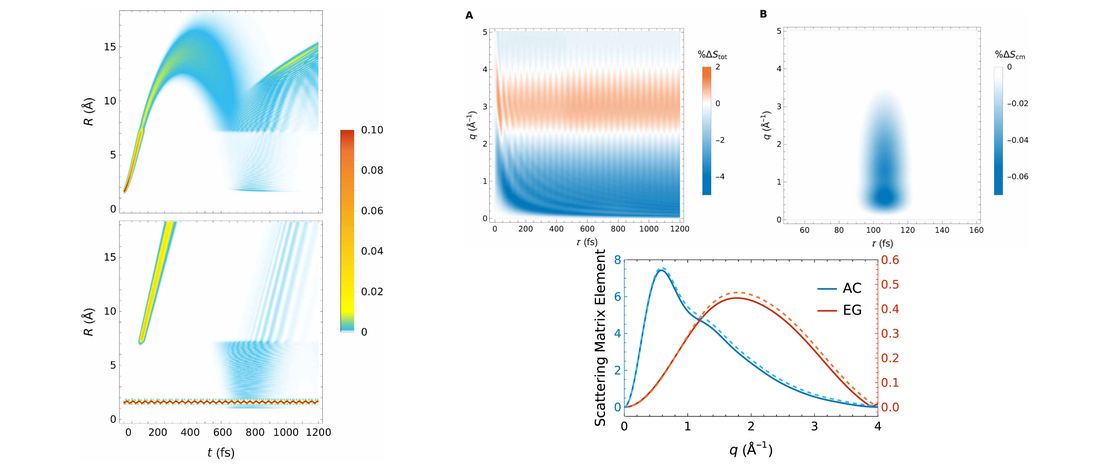
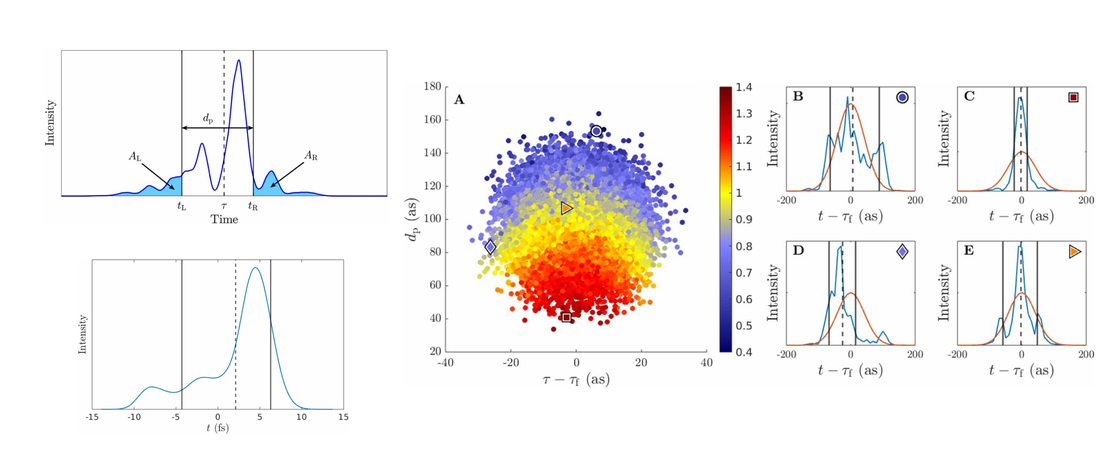
X-ray free electron lasers (XFELs) have emerged as powerful sources of short and intense x-ray pulses. We propose a simple and robust procedure which takes advantage of the inherent stochasticity of self-amplified stimulated emission (SASE) pulses to enhance the time-resolution and signal strength of the recorded data. Notably, the proposed method is able to enhance the average signal without knowledge of the signal strength of individual shots. Simple metrics for the probe pulses are introduced, such as an effective pulse duration applicable to SASE pulses characterised in the time domain using e.g. an X-band transverse cavity. The approach is evaluated using simulated and real pulse data in the context of ultrafast electron dynamics in a molecule. Utilising H2 as a model system, we demonstrate the efficacy of the method theoretically, successfully enhancing the predicted nonresonant ultrafast x-ray scattering signal associated with electron dynamics. The method presented is broadly applicable and offers a general strategy for enhancing time-dependent observables at XFELs.
Additional Resources
DOI: 10.1088/1361-6455/ad8a33
Bibtex:
@article{lia24xfel,
doi = {10.1088/1361-6455/ad8a33},
url = {https://dx.doi.org/10.1088/1361-6455/ad8a33},
year = {2024},
month = {nov},
publisher = {IOP Publishing},
volume = {57},
number = {23},
pages = {235605},
author = {Eirik M Liane and Mats Simmermacher and Peter M Weber and Adam Kirrander},
title = {XFEL SASE pulses can enhance time-dependent observables},
journal = {Journal of Physics B: Atomic, Molecular and Optical Physics},
}